9+ Chapter 17 Thermochemistry Answer Key
An acid-base reaction is one in which a hydrogen ion H is transferred from one chemical species to anotherSuch reactions are of central importance to numerous natural and technological processes ranging from the chemical transformations that take place within cells and the lakes and oceans to the industrial. Download Free PDF View PDF.

Chapter 17 Review Thermochemistry Ppt Download
Web By the twentieth century it became apparent that the periodic relationship involved atomic numbers rather than atomic masses.
. The larger the K a of an acid the larger the concentration of H 3 O H 3 O and A relative to the concentration of the nonionized acid HA in an equilibrium mixture and the stronger the. Web Ozone ˈ oʊ z oʊ n or trioxygen is an inorganic molecule with the chemical formula O 3It is a pale blue gas with a distinctively pungent smell. You confirm the marks.
This can be explained with the concept of effective nuclear charge Z eff. Answer Groups AI-assisted Grading Grade groups of similar answers at once. Keep in mind of course that the formula subscripts define in part the identity of the substance and so these cannot be changed without altering the qualitative.
The amount of product that may be produced by a reaction under specified conditions as calculated per the stoichiometry of an appropriate balanced chemical equation is called the theoretical yield of the reaction. Practice and Study Guide TExES Mathematics 7-12 235 Prep. Web For example phenolphthalein is a colorless substance in any aqueous solution with a hydronium ion concentration greater than 50 10 9 M pH 83.
In this case however masses not molar amounts are provided and requested so additional steps of. Web As shown in Figure 631 as we move across a period from left to right we generally find that each element has a smaller covalent radius than the element preceding itThis might seem counterintuitive because it implies that atoms with more electrons have a smaller atomic radius. The Ideal Gas Law.
The properties of the elements are periodic functions of their atomic numbersA modern periodic table arranges the elements in increasing order of their. The Ideal Gas Law. Web Let us guess that the abundances are 9 Ne-22 91 Ne-20 and only a trace of Ne-21.
Web CHAPTER 15 ACIDS AND BASES. That is we must derive an appropriate stoichiometric factor from the balanced chemical equation and use it to relate the amounts of the two substances of interest. Download Free PDF View PDF.
Download Free PDF View PDF. In addition the technologies used to extract work from a spontaneous process eg batteries are never 100 efficient and so the work done by these processes is always less than the. Export Grades Analytics.
In practice the amount of product obtained is called the actual yield and it is often less than the theoretical yield for a. 95 The Kinetic-Molecular Theory. Dilithium monoxide would be the name if Li2O were a covalent compound a compound composed of only nonmetals.
10 Qs 515 plays. 92 Relating Pressure Volume Amount and Temperature. Web 91 Gas Pressure.
Although water is a reactant in the reaction it is the solvent as well so we do not include H 2 O in the equation. Web Solution The approach used previously in Example 48 and Example 49 is likewise used here. It is an allotrope of oxygen that is much less stable than the diatomic allotrope O 2 breaking down in the lower atmosphere to O 2 Ozone is formed from dioxygen by the action of ultraviolet UV light and electrical.
Web 17 Qs 439 plays. 15 slides 606 plays. Download Free PDF View PDF.
Web An earlier chapter of this text introduced solutions defined as homogeneous mixtures of two or more substancesOften one component of a solution is present at a significantly greater concentration in which case it is called the solventThe other components of the solution present in relatively lesser concentrations are called solutesSugar is a covalent solid. American Revolution Battlegrounds Shift. Web where the concentrations are those at equilibrium.
However as noted previously in this chapter such conditions are not realistic. ATOMS MOLECULES AND IONS. 92 Relating Pressure Volume Amount and Temperature.
American Revolution The Final Years of the War. 13 Qs 429 plays. In more basic solutions where the hydronium ion concentration is.
Web When performing calculations stepwise as in Example 317 it is important to refrain from rounding any intermediate calculation results which can lead to rounding errors in the final resultIn Example 317 the molar amount of NaCl computed in the first step 1325 mol would be properly rounded to 132 mol if it were to be reported. Simple Compound and Complex Sentences. 15 Qs 519 plays.
The average mass would be 2018 amu. Web where w max w max refers to all types of work except expansion pressure-volume work. For some question types Gradescope AI automatically forms.
To achieve balance the coefficients of the equation may be changed as needed. Checking the natures mix of isotopes shows that the abundances are 9048 Ne-20 925 Ne-22 and 027 Ne-21 so our guessed amounts have to be slightly adjusted. Web x 13 10 6 4 3 69 10 3 M x 13 10 6 4 3 69 10 3 M As defined in the ICE table x is the molarity of calcium ion in the saturated solution.
94 Effusion and Diffusion of Gases. Web 91 Gas Pressure. 93 Stoichiometry of Gaseous Substances Mixtures and Reactions.
The modern statement of this relationship the periodic law is as follows. The rate of reaction is solely dependent on the concentration of NO 2A later chapter section on reaction mechanisms will explain how a reactants concentration can have no effect on a reaction. An example of experimental pressure-temperature data is shown for a sample of air under these conditions in Figure 911We find that temperature and pressure are linearly related and if the temperature is on the kelvin scale then P.
15 Qs 56k plays. 94 Effusion and Diffusion of Gases. The dissolution stoichiometry shows a 11 relation between moles of calcium ion in solution and moles of compound dissolved and so the molar solubility of CaOH 2 is.
Web This relationship between temperature and pressure is observed for any sample of gas confined to a constant volume. Web Bubble sheets are auto-graded according to your answer key. Web FTCE Middle Grades General Science 5-9 004 Prep MCAT Test.
Web The numbers of H atoms on the reactant and product sides of the equation are equal but the numbers of O atoms are not. 95 The Kinetic-Molecular Theory. Web Remember that a number raised to the zero power is equal to 1 thus CO 0 1 which is why the CO concentration term may be omitted from the rate law.
Web We would like to show you a description here but the site wont allow us. Send grades to your students or gradebook and view item analysis data and other statistics. 20 Qs 2k plays.
96 Non-Ideal Gas Behavior. 96 Non-Ideal Gas Behavior. Because lithium is assumed to form 1 ions in compounds we do not need to indicate the charge of the metal ion in the compound.
Practice and Study Guide TExES Mathematics 7-12 235 Prep. Web FTCE Middle Grades General Science 5-9 004 Prep MCAT Test. 93 Stoichiometry of Gaseous Substances Mixtures and Reactions.
Web Chemistry Answer Ion Chemical Bond.

Language Of Chemistry Worksheet

Pdf Reactions And Thermochemistry Of Alkyl Ions Cnh2n 1 N 1 8 In The Gas Phase

Chapter 17 Review Answers

Chapter 17 Review Thermochemistry Ppt Download

Chapter 17 Thermochemistry Ppt Video Online Download

Chapter 17 Thermochemistry
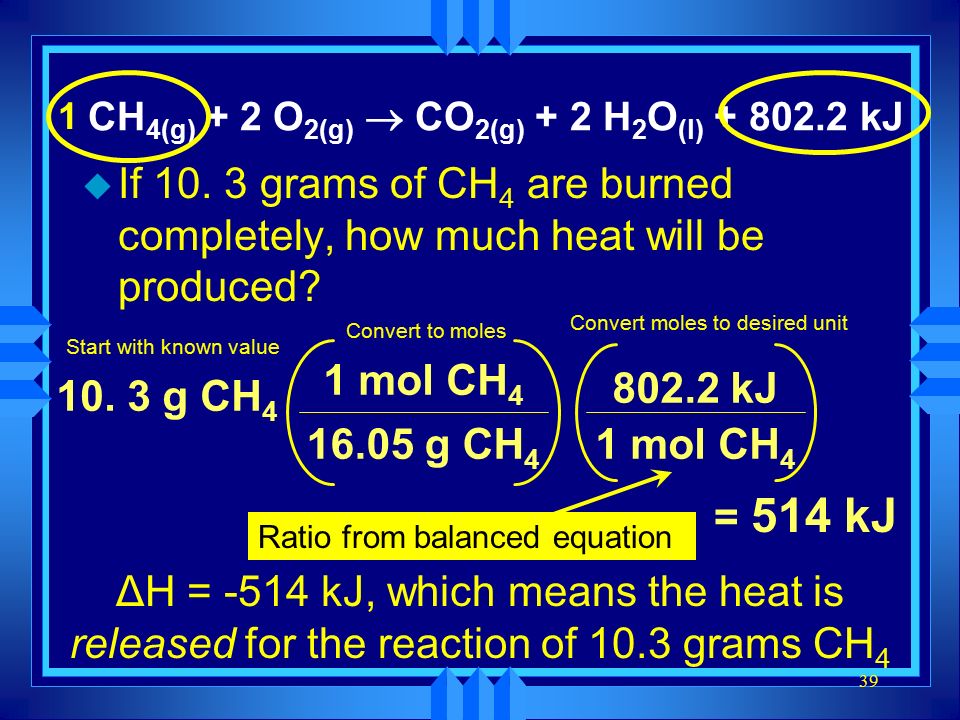
Chapter 17 Thermochemistry Ppt Video Online Download

Chem 1051 Mini Assignment On Chemical Equilibrium Practical Chem 1051 Mini Assignment On Studocu
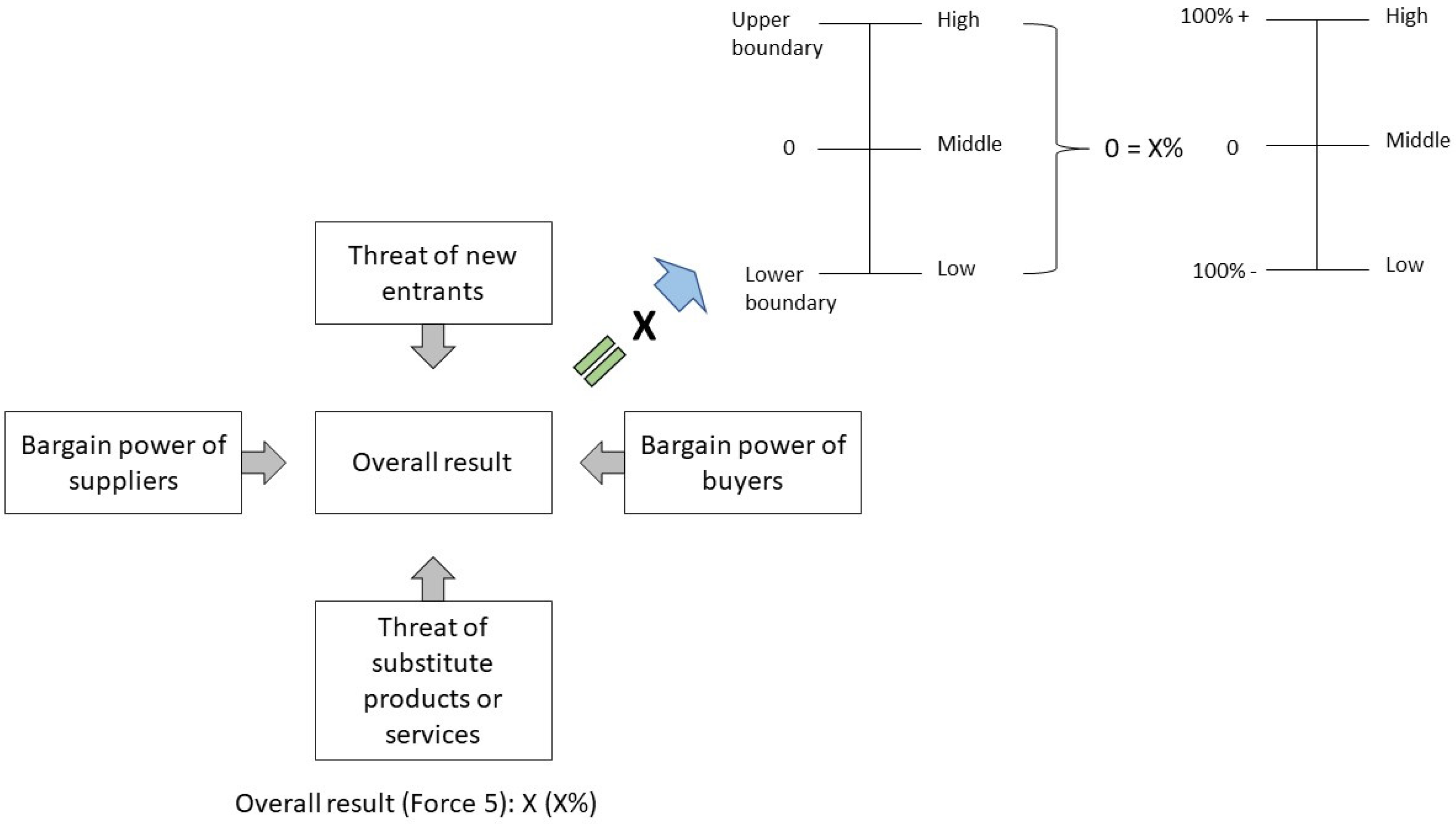
Sustainability Free Full Text Potential Evolution Of The Cooling Market In The Eu27 Uk An Outlook Until 2030 Html

Language Of Chemistry Worksheet

Ab Initio Calculations Springerlink

A Simple Chemical Tuning Of The Effective Concentration Selection Of Single Double And Triple Stranded Binuclear Lanthanide Helicates Terazzi 2009 Chemistry A European Journal Wiley Online Library

Chapter 17 Thermochemistry Ppt Video Online Download

Chapter 17 Thermochemistry

Chapter 17 Thermochemistry

Pdf Towards An Aviation Fuel Through The Hydrothermal Liquefaction Of Algae
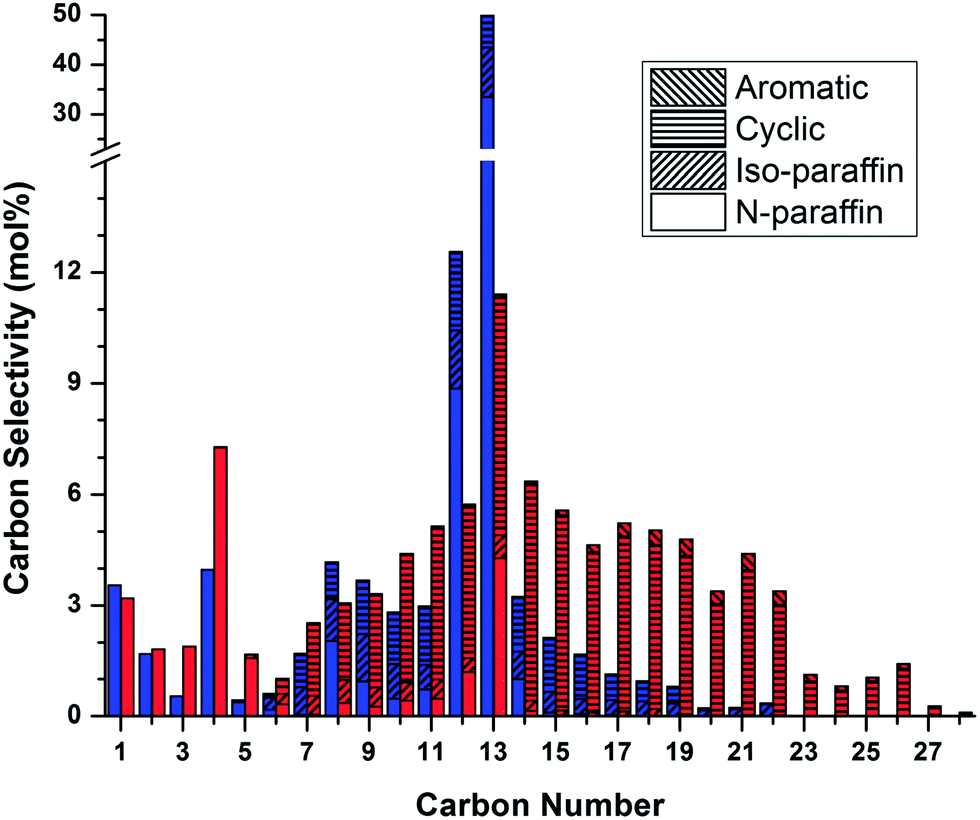
Production Of Renewable Jet Fuel Range Alkanes And Commodity Chemicals From Integrated Catalytic Processing Of Biomass Energy Environmental Science Rsc Publishing Doi 10 1039 C3ee43846e